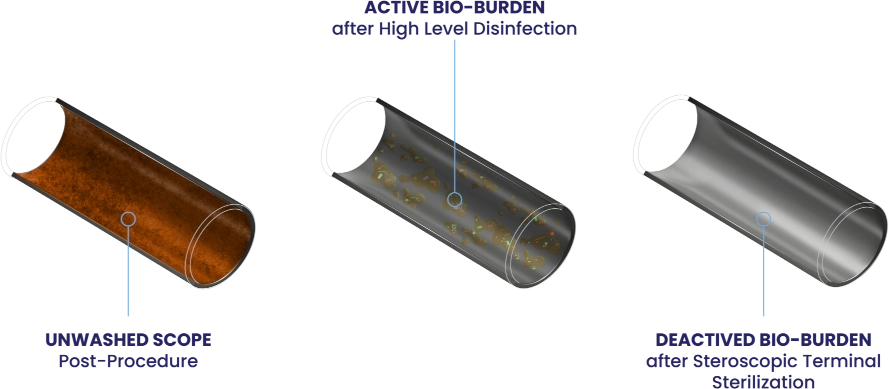
“The margin of safety associated with reprocessing endoscopes is minimal. Endoscopes are heavily contaminated with microbes. The internal channel of gastrointestinal endoscopes may contain 10⁸–10¹⁰ enteric microorganisms.”
- JAMA “A Need to Shift from Disinfection to Sterilization”